¡Órale! 18+ Raras razones para el Labeled Diagram Of An Atom! We say the electrons ‘reside’ in different principal energy.
Labeled Diagram Of An Atom | We say the electrons 'reside' in different principal energy. This is the stage where dna replication is initiated. This model is even more approximate than the model of hydrogen, because it treats the electrons in each shell as … Inertia describes the relative amount of resistance to change that an object possesses. The position of the isotopes in the products is measured to …
The position of the isotopes in the products is measured to … The excited atoms do not all emit the same energy light because the amount of energy that excited them may differ, but there are limitations on the colors they do emit. Inertia describes the relative amount of resistance to change that an object possesses. This model is even more approximate than the model of hydrogen, because it treats the electrons in each shell as … This figure is a diagram of a short stretch of a dna molecule which is unwound and flattened for clarity.
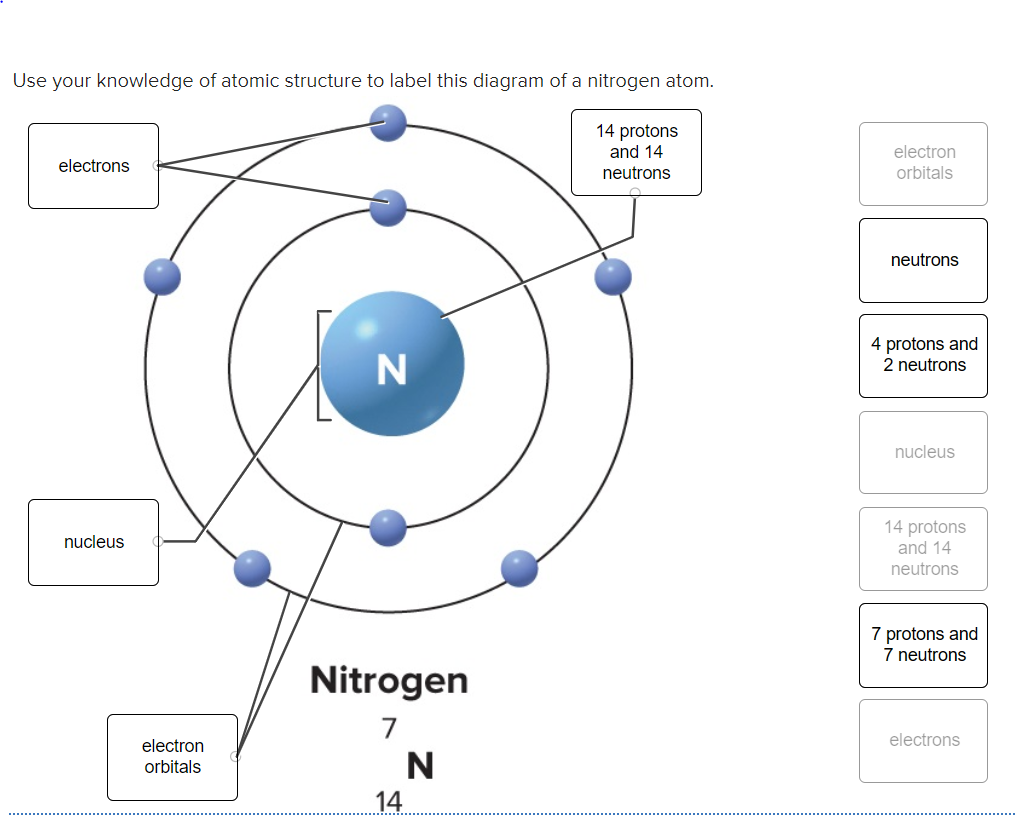
The volume of a nucleus was considered to be negligible compared to the … Dna synthesis is initiated within the template strand at a specific coding region site known as origins.; An atom consists of a positively charged nucleus and negatively charged electrons. The position of the isotopes in the products is measured to … According to his atom diagram, the atom has a small, positively charged nucleus in center. The excited atoms do not all emit the same energy light because the amount of energy that excited them may differ, but there are limitations on the colors they do emit. The reactant is then allowed to undergo the reaction. The electrostatic attraction between them keeps electrons 'bound' to the nucleus so they stay within a certain distance of it. Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell.the reactant is 'labeled' by replacing specific atoms by their isotope. But not all objects accelerate at the same rate when exposed to the same amount of unbalanced force. There are so many pulses emitted the light appears to be continous. This model is even more approximate than the model of hydrogen, because it treats the electrons in each shell as … And when that happens, it's double trouble for physics students.
The excited atoms do not all emit the same energy light because the amount of energy that excited them may differ, but there are limitations on the colors they do emit. An atom consists of a positively charged nucleus and negatively charged electrons. The reactant is then allowed to undergo the reaction. Each nucleotide is itself make of three subunits: According to his atom diagram, the atom has a small, positively charged nucleus in center.

Inertia describes the relative amount of resistance to change that an object possesses. Careful investigations have shown that not all electrons within an atom have the same average position or energy. There are so many pulses emitted the light appears to be continous. Dna synthesis is initiated within the template strand at a specific coding region site known as origins.; The excited atoms do not all emit the same energy light because the amount of energy that excited them may differ, but there are limitations on the colors they do emit. An atom consists of a positively charged nucleus and negatively charged electrons. Each nucleotide is itself make of three subunits: But not all objects accelerate at the same rate when exposed to the same amount of unbalanced force. One arrow (labeled "a" in the diagram) that starts at a lone pair of electrons on the basic oxygen atom of the hydroxide anion, then points to the acidic h atom of acetic acid to indicate formation of the new bond being made. Unbalanced forces cause objects to accelerate. 31.03.2005 · each excited atom releases a single pulse of light energy as it returns to the ground state or low energy state. The boxed area at the lower left encloses one nucleotide. And when that happens, it's double trouble for physics students.
The boxed area at the lower left encloses one nucleotide. This is the stage where dna replication is initiated. The greater the mass the object possesses, the more inertia that it has, and the greater its tendency to not accelerate as much. The excited atoms do not all emit the same energy light because the amount of energy that excited them may differ, but there are limitations on the colors they do emit. A five carbon sugar called deoxyribose (labeled s) a phosphate group (a phosphorous atom surrounded by four oxygen atoms.) (labeled p) and one of four nitrogen …
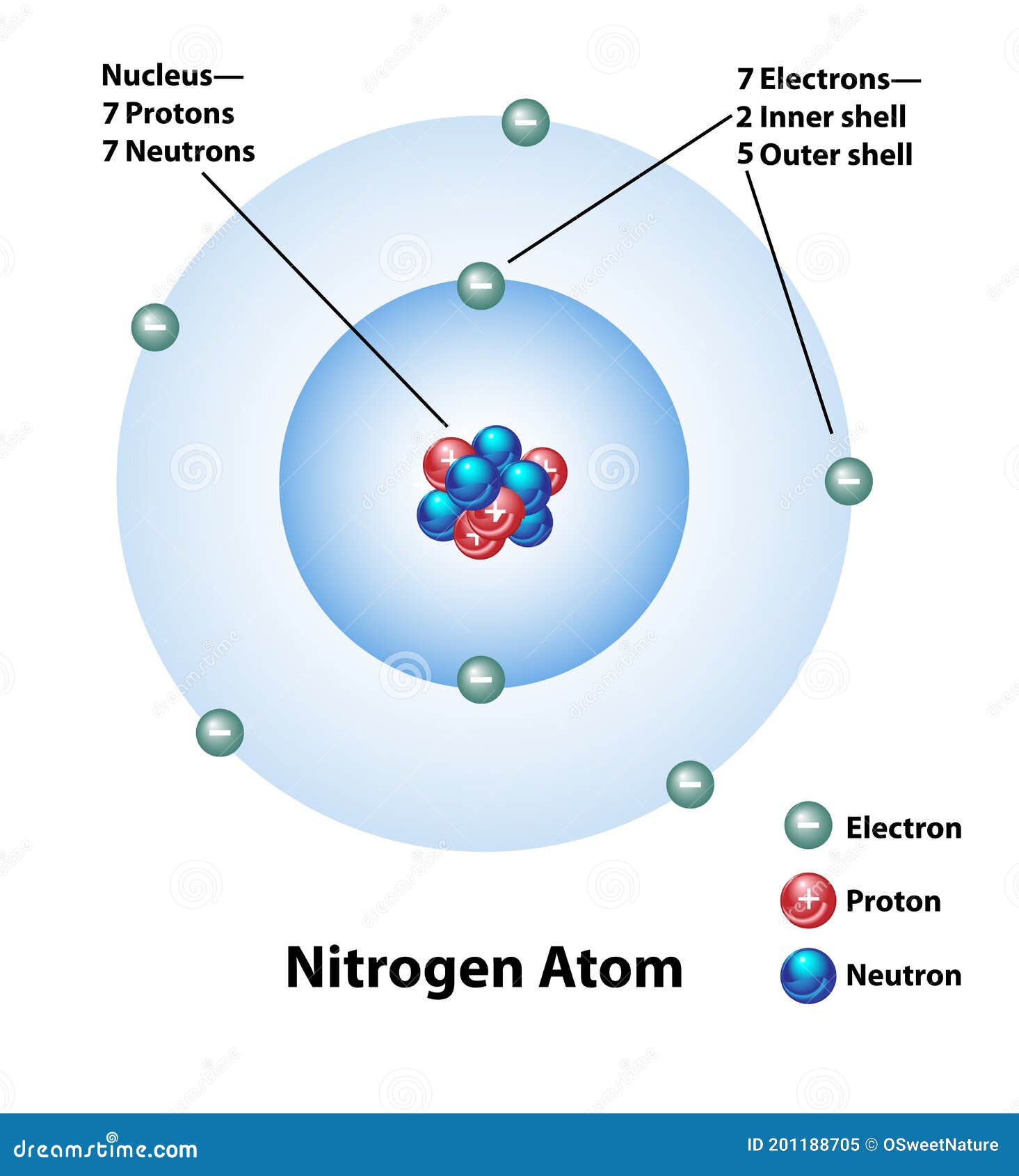
Inertia describes the relative amount of resistance to change that an object possesses. Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope (an atom with a detectable variation in neutron count) through a reaction, metabolic pathway, or cell.the reactant is 'labeled' by replacing specific atoms by their isotope. A five carbon sugar called deoxyribose (labeled s) a phosphate group (a phosphorous atom surrounded by four oxygen atoms.) (labeled p) and one of four nitrogen … The reactant is then allowed to undergo the reaction. We say the electrons 'reside' in different principal energy. An atom consists of a positively charged nucleus and negatively charged electrons. 31.03.2005 · each excited atom releases a single pulse of light energy as it returns to the ground state or low energy state. The number of electrons were equal to the number of protons and therefore, an atom is electrically neutral. This figure is a diagram of a short stretch of a dna molecule which is unwound and flattened for clarity. Dna synthesis is initiated within the template strand at a specific coding region site known as origins.; Careful investigations have shown that not all electrons within an atom have the same average position or energy. There are so many pulses emitted the light appears to be continous. The boxed area at the lower left encloses one nucleotide.
A five carbon sugar called deoxyribose (labeled s) a phosphate group (a phosphorous atom surrounded by four oxygen atoms) (labeled p) and one of four nitrogen … labeled diagram of an. The excited atoms do not all emit the same energy light because the amount of energy that excited them may differ, but there are limitations on the colors they do emit.
Labeled Diagram Of An Atom! And when that happens, it's double trouble for physics students.
0 Response to "¡Órale! 18+ Raras razones para el Labeled Diagram Of An Atom! We say the electrons ‘reside’ in different principal energy."
Post a Comment